Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Machine Learning Predictions of COPD Development and Physiological Responses in Smoke-Inhaled Lab Rats Using CNN Architectures
Authors: Vinay Kumar Tripathi, Shubham Tripathi
DOI Link: https://doi.org/10.22214/ijraset.2024.63794
Certificate: View Certificate
Abstract
Globally, Chronic Obstructive Pulmonary Disease (COPD) is a major public health concern that necessitates the use of efficient predictive technologies for early detection and treatment. Using convolutional neural network (CNN) architectures—a recent development in machine learning—this study investigates the physiological responses and COPD development prediction in lab rats exposed to smoking. Three well-known CNN algorithms— ResNet, GoogleNet, and VGG19—were used to examine data gathered from studies in which lab rats were exposed to smoke over time. Throughout the trial, physiological parameters and the evolution of COPD were observed. Our findings show how effective these CNN architectures are at predicting the onset of COPD and recognising the physiological reactions linked to smoking. The application of the VGG19, ResNet, and GoogleNet algorithms advances our understanding of COPD prediction in experimental environments and opens up new avenues for future study focused on improving therapeutic interventions and early detection techniques. Keywords— COPD, machine learning, CNN architectures, smoke exposure, experimental study.
Introduction
I. INTRODUCTION
The complexity of COPD is a major barrier to successful therapy, notwithstanding advancements in our knowledge of the disease's pathophysiology and the identification of risk factors. There is a wide range of phenotypes and endotypes associated with COPD, each having unique clinical characteristics, disease paths, and treatment outcomes. Conventional diagnostic standards, including forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), which rely on spirometry data, might not account for this intricacy, misclassifying patients and resulting in less-than-ideal treatment plans.This problem may be solved by machine learning techniques, which combine multidimensional data and use advanced algorithms to find hidden patterns and subgroups among COPD populations. Personalised treatment decisions and improved outcomes for COPD patients can be facilitated by machine learning algorithms that identify discrete phenotypic clusters and characterise their specific clinical profiles.
Apart from improving diagnostic precision and risk assessment, machine learning exhibits potential in forecasting exacerbations of COPD and directing preventive measures. Effective preventative methods are necessary since COPD exacerbations are linked to faster disease progression, a lower quality of life, and more healthcare utilisation. Through the examination of longitudinal data from patients with COPD, which includes clinical characteristics, physiological measurements, and environmental factors, machine learning algorithms are able to predict with high accuracy when and how exacerbations will occur.In addition, machine learning algorithms are able to pinpoint individualised intervention methods and modifiable risk factors that reduce the likelihood of exacerbations. Examples of these efforts include enhancing medication compliance, encouraging quitting smoking, and offering focused respiratory rehabilitation programmes. Proactive management strategies based on predictive analytics can help healthcare practitioners reduce the number of COPD exacerbations and enhance patients' long-term results.
In conclusion, machine learning offers unmatched capabilities in data analysis, predictive modelling, and personalised treatment, hence representing a paradigm shift in COPD research and clinical practice. Researchers and doctors may use the power of big data to better understand the pathophysiology of COPD, increase diagnosis accuracy, tailor treatments, and improve patient outcomes by utilising machine learning techniques. We can fully utilise machine learning to handle the complex problems presented by COPD and open the door to a new era of precision respiratory treatment by working together across disciplines, such as computer science, biomedical engineering, and respiratory medicine.
II. BACKGROUND STUDY
Cigarette smoke was chronically introduced to male Wistar rats by Duarte et al. (2005) [1]. The vocal folds' histological examination showed hyperplasia, squamous metaplasia, and keratinizing metaplasia, all of which pointed to cellular injury. These results highlight the significance of tobacco control efforts and the necessity to comprehend how susceptible the voice cords are to secondhand smoke exposure. Using Sprague-Dawley rats, Baek et al. (2022) [2] used the Mask R-CNN method to identify lesions associated with acute hepatic damage caused by acetaminophen. At the whole-slide image level, the model identified lesions with 96.44% accuracy. The algorithm's ability to predict hepatic lesions was proved by the excellent correlation it showed when compared to expert-annotated areas. The paper recommends the Mask R-CNN method as a useful resource for identifying liver abnormalities in investigations that are not clinical; potential applications exist in preclinical and clinical contexts.In Sprague-Dawley rats, Iida et al. (1998) [3] examined the effects of nicotine infusion and cigarette smoking on the cerebrovascular system. They evaluated pial vessel diameters using a prepared cranial window and compared single-cigarette smoking, repeated smoking, and nicotine infusion. Biphasic effects were caused by smoking a single cigarette; vasoconstriction came first and then vasodilation. Vasodilation was decreased but not vasoconstriction by repeated smoking. Without first causing vasoconstriction, nicotine infusion resulted in vasodilation. Pharmacological interventions demonstrated that thromboxane A2, NO generation, sympathetic activation, and K1 channel activation were all involved in the effects that were observed. These results clarify the intricate processes that underlie the effects of nicotine infusion and cigarette smoking on the cerebrovascular system in rats.et al. Barnard (2023) [4] investigated the impact of high-THC cannabis smoking on male rats' incidental recall. They discovered that THC smoke affected odour recognition under both memory loads and object recognition under high memory load using novelty preference testing. These results imply that cannabis smoking has stimuli-specific and memory load-dependent effects on rat memory.Church and Pryor (1985) [5] investigated the toxicological effects of cigarette smoke's free-radical chemistry. They found that there were two different kinds of radicals in cigarette smoke: highly reactive radicals in the gas phase and stable radicals in the tar phase, primarily a quinone/hydroquinone (Q/QH2) complex. These radicals can oxidise thiols and react with DNA. The study sheds light on the complex effects of cigarette smoke on lipid peroxidation and its role in radical-mediated disease processes. Wright and Churg (2008) [6] reviewed the use of animal models in chronic obstructive pulmonary disease (COPD) research, highlighting their value and potential limitations. Animal models offer valuable insights into COPD pathophysiology and therapeutic development, allowing researchers to control conditions and exposures precisely. These models enable the study of single or combined insults, such as tobacco smoke or allergen exposure, providing a platform to investigate disease mechanisms and test potential therapies. However, the choice of model requires careful consideration to ensure relevance and translational validity to human COPD.
Proteomics approaches have been applied to the identification of biomarkers for chronic lung disorders, such as COPD and asthma, as reviewed by Haenen et al. (2014) [7]. The phenotypes of these disorders are highly variable, which makes the identification of biomarkers difficult. Proteomics has a high throughput and sensitivity, which makes it a potential method for finding biomarkers in animal and human models. To find biomarkers for asthma and COPD, the authors reviewed the literature on proteomics research carried out in human and murine models. Although mouse models have been developed and proteomics research has been conducted, there is still minimal translation of potential biomarkers into therapeutic trials.Liang and associates (2019) [8] reviewed the development, assessment techniques, and recommendations for enhancements for animal models of COPD and emphysema. They draw attention to how important these models are for comprehending respiratory illnesses and provide ideas for further study and treatment approaches. The relationship between smoking cigarettes and idiopathic pulmonary fibrosis (IPF), which is linked to emphysema, was examined by Qiao et al. (2014) [9]. They examined the differences in IPF patients with and without emphysema's clinical characteristics, smoking histories, and radiological results. Their research adds to our understanding of the clinical features of this combination illness by highlighting the importance of smoking as a major risk factor.Li et al. suggested a rat model for repeated bacterial infection (RBI) and cigarette smoke inhalation (CSI)-induced chronic obstructive pulmonary disease (COPD). Rats in the study were given either CSI, RBI, or a combination of both, and then they were studied for 20 weeks. The COPD rats' results revealed reduced lung function, elevated cytokine levels, and histological alterations; the combination therapy group showed the strongest effects. The combined CSI and RBI treatment, according to the researchers, may provide a better model for researching human COPD.Zhang and others. [11] identified individuals with high-risk chronic obstructive pulmonary disease (COPD) using quantitative computed tomography (QCT) readings. Based on results from pulmonary function testing, 140 patients were enrolled and divided into low- and high-risk COPD groups. High-risk COPD was identified with 85.71% accuracy using SVM analysis. When QCT and clinical data were combined, accuracy increased to 90.48%. PFT results and QCT indexes showed a correlation, suggesting the utility of QCT indexes in determining the severity of COPD.Tanabe et al.
[12] evaluated the viability of quantitative assessments of smaller airways in chest ultra-high- resolution computed tomography (U-HRCT) utilising deep learning- based reconstruction, Advanced intelligent Clear-IQ Engine (AiCE). AiCE preserved image quality while reducing reconstruction time by about 90% in comparison to the full-iterative model. AiCE-based U- HRCT yielded results that were on par with or better than phantom tube experiments and patient studies when assessing tiny airways, indicating its potential for clinical use in assessing peripheral airways.
Over a three-month period, Konietzke et al. [13] tracked short-term changes in individuals with severe COPD using quantitative CT (QCT). While air-trapping stayed constant, emphysema metrics increased dramatically. PRM showed less normal lung tissue and increased emphysema. Heterogeneous alterations were observed in airway measurements. Beyond spirometry, QCT provides insights into COPD heterogeneity by accurately detecting the advancement of emphysema.A technique for accurately detecting COPD phases was established by Deng et al. [14] using inspiratory and expiratory chest CT images. Their method surpassed traditional machine learning classifiers, with an accuracy of 89.7%. This technique is a useful diagnostic tool that shows promise for accurate COPD diagnosis.Yang et al. [15] presented a technique for auto-metric graph neural network (AMGNN) and lung radiomics features-based COPD stage classification. They used the GLM and Lasso algorithms to create a lung radiomics combination vector, which resulted in a good classification performance (accuracy: 94.3%). Their method proved successful in COPD stage classification, outperforming previous neural networks and machine learning algorithms. In order to avoid severe emphysema, Jung and Vij [16] emphasise the criticality of early COPD diagnosis and real-time monitoring. They draw attention to the main causes and stress the importance of prompt therapies to maintain lung function. Due to the limitations of current diagnostic approaches, research is being done on cutting edge methods including X-ray phase contrast and machine learning for better monitoring and detection. They support the use of cutting-edge diagnostics to facilitate prompt therapies and stop the progression of COPD. [17] describes how ageing populations and tobacco usage are contributing to the increased prevalence of COPD and emphasises quitting smoking as the primary preventive measure. Lung function gradually deteriorates despite an early, frequently symptom-free phase, forcing patients to seek care in later stages or during exacerbations. Devine emphasises the significance of a precise diagnosis and ongoing care to maintain quality of life and lower COPD-related medical expenses. The association between the disease load and COPD symptoms is examined by Miravitlles et al. [18]. This relationship takes into account a number of factors, including prognosis, anxiety, depression, daily activities, physical activity, quality of life, and health status. They draw attention to how different symptoms can be felt during the day and at night. Their narrative review, which primarily draws on observational and real- world studies, highlights the important role that COPD symptoms play in the overall disease burden and stresses the significance of identifying and comprehending this role for efficient therapy.In their examination of the prevalence and future prospects of chronic obstructive pulmonary disease (COPD), Lopez et al. (2006) [19] stress the significance of precise epidemiological data. They state that there were over 2.7 million COPD-related fatalities globally in 2000, with a large share of those deaths taking place in the Western Pacific Region, particularly China. Additionally, industrialised nations reported almost 400,000 fatalities per year from COPD. The incidence varied by region, from 3-5 percent in some parts of Africa to 2-4 percent in North America. The study emphasises the necessity of standardised procedures and thorough data in order to address the increasing impact of COPD on public health.
III. METHODOLOGY
This study's experimental design was painstakingly created to look at how lab rats exposed to smoking develop Chronic Obstructive Pulmonary Disease (COPD) and its physiological effects. For varied amounts of time—from brief to extended exposure periods—lab rats were exposed to regulated volumes of smoke produced by ordinary cigarette smoke. The smoke inhalation approach allowed for the progressive emergence of COPD-related symptoms and physiological changes over time. It was aimed to replicate the chronic exposure patterns seen in human smokers.
A. Dataset
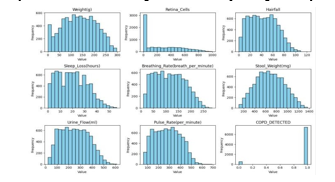
This study's dataset was painstakingly created to examine the physiological effects of gas exposure on lab rats and assess the emergence of Chronic Obstructive Pulmonary Disease (COPD) as a result of prolonged exposure times. It includes a variety of physiological measures that are carefully documented in lab rats that have inhaled smoke over time, as well as a binary indication that indicates the presence of COPD.The dataset includes a wide range of characteristics designed to record different physiological reactions and health status markers in the exposed rats.
These factors include weight in grammes, the measurement of retina cells to estimate possible effects on the eyes, and the evaluation of hairfall as a sign of toxicity or systemic stress, assessment of the length of sleep loss as a gauge of post-exposure sleep disturbances, heart rate assessments to track cardiovascular responses, stool weight quantification to evaluate gastrointestinal effects, respiratory rate measurements to track lung function, urine flow rate measurement to gauge renal function and hydration status, and a binary variable based on diagnostic criteria and histopathological examination to indicate the presence or absence of COPD.
Strict guidelines were followed during the data collection process to guarantee accuracy and consistency. The lab rats that were exposed to carefully regulated gas inhalation regimens and smoke were subjected to a series of systematic measurements. To capture longitudinal changes in physiological parameters and the development of COPD, data points were painstakingly captured at specified intervals throughout successive time periods. This allowed for a thorough evaluation of the impact of gas exposure on the rats' health state.The dataset was carefully preprocessed to improve its quality and enable accurate model performance before training and analysis. In order to maximise model convergence and forecast accuracy, this involved addressing missing values, identifying and managing outliers, and normalising feature scales.Moreover, in order to promote transparency, the produced dataset will be publicly available to the scientific community together with thorough metadata and annotations, promoting reproducibility and cooperative research activities. Researchers should get in contact with the corresponding author for more details and possible collaboration opportunities if they are interested in accessing the dataset or duplicating the study methods.
B. Models
This paper used three different convolutional neural network (CNN) architectures to analyse the dataset and forecast the development of Chronic Obstructive Pulmonary Disease (COPD) and the physiological reactions of smoke-inhaled lab rats. VGG19, ResNet, and GoogleNet are the three CNN architectures that were chosen because of their distinct architectural characteristics and aptitude for managing complex data.
Within the field of convolutional neural networks (CNNs), VGG19 is a benchmark known for its resilience and ease of use. There are 19 layers in total in this architecture, most of which are 3x3 convolutional filters with max-pooling layers dotted throughout the network. Its architecture is characterised by a simple, uncomplicated design that makes training and understanding easier. VGG19 has a more complex structure than VGG16, but it still has a clear and consistent design, which makes it a good option for a variety of image processing applications, especially image classification. VGG19's unique layer-by-layer development makes it possible to extract features in a hierarchical manner, which helps the network identify complex patterns and correlations in the input data.. VGG19 was used in this work to evaluate the dataset and forecast results pertaining to the onset of COPD and physiological reactions. VGG19 was assigned the responsibility of deriving meaningful representations from the input features, utilising its depth and simplicity in architecture to contribute to the thorough evaluation of COPD-related outcomes.ResNet, short for Residual Network, introduced residual connections, also known as skip connections, in a way that marked a major advancement in CNN topologies. By learning residual mappings thanks to these connections, the network is able to avoid the vanishing gradient issue that arises when training incredibly deep networks. Because of their unparalleled depth and effectiveness, ResNet designs, including ResNet50 and ResNet101, have become industry mainstays in the fields of image categorization and feature extraction. ResNet topologies can capture subtle features and patterns with surprising precision by exploring deeper into the data hierarchy and leveraging residual connections. ResNet was used in this work to analyse the dataset and identify outcomes associated to COPD. Its sophisticated architectural design was utilised to help understand the subtleties of physiological reactions to smoke inhalation.
Google's DeepMind team designed GoogleNet, dubbed Inception- v3, which is the ultimate CNN design. Its essential component is the inception module, which combines several simultaneous convolutional operations with different filter sizes in a clever way. This architecture improves GoogleNet's ability to extract features and learn representations by allowing it to extract both local and global characteristics from the input data. Through the use of this multi- scale methodology, GoogleNet is able to surpass the constraints of conventional CNN architectures in its ability to identify complex patterns and structures in the input data. GoogleNet's exceptional performance and versatility have won it praise in the image identification space, where its efficiency and computational prowess have thrust it to the forefront. GoogleNet was given the job of analysing the dataset for this investigation and forecasting outcomes pertaining to COPD development and projecting results regarding the onset of COPD and physiological reactions. By utilising its creative architectural layout and multi-scale feature extraction powers, GoogleNet helped to further the thorough investigation of COPD- associated results in lab rats exposed to smoke.
C. Model Training and Evaluation
Using the supplied dataset, each CNN architecture was trained using a supervised learning methodology. The dataset was carefully divided into training, validation, and test sets to enable repeated model training and performance evaluation, ensuring robust model development. Stochastic gradient descent (SGD) and Adam optimisation were used to optimise the model's parameters during the training phase, hence improving the model's capacity to identify significant patterns in the data. In order to improve prediction accuracy and generalisation to new data, the model's parameters were iteratively adjusted during the training phase with the goal of minimising the model's loss function.Following the training phase, the model's performance was thoroughly assessed using the aforementioned F1-score in addition to common evaluation measures like recall and accuracy. These measures gave us information on how well the model identified cases of COPD development and reduced the number of false positives. Accuracy evaluated the overall correctness of the model's predictions across all classes, whereas recall rated the model's sensitivity in identifying true positive occurrences of COPD. When taken as a whole, these measures provided a thorough assessment of the model's predictive power and potential to aid in the evaluation of COPD-related outcomes.
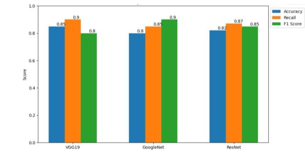
To compare the three CNN architectures' performance across several evaluation parameters, statistical studies were carried out. To evaluate overall differences in model performance, ANOVA tests were used to determine whether the differences between the topologies were statistically significant. After that, pairwise comparisons between each model were carried out using post-hoc tests like Tukey's HSD in order to clarify particular performance differences. The outcomes of these statistical analyses gave important information about the relative advantages and disadvantages of each CNN design, which helped with model selection and improvement decisions.Bar graphs were used as visual representations to show how each model performed across several evaluation metrics, which improved the results' interpretability. With the use of these graphs, stakeholders were able to quickly identify patterns and trends by seeing a clear and understandable representation of the models' relative performance. To provide a thorough overview of the performance variations between the models and aid in decision-making and future research areas, a comparison chart was also created.
Conclusion
In this work, we used convolutional neural network (CNN) architectures to conduct a thorough investigation of machine learning predictions of the development of Chronic Obstructive Pulmonary Disease (COPD) and physiological responses in smoke-inhaled lab rats. Using a carefully selected dataset with several physiological parameters, we used three well-known CNN architectures (VGG19, ResNet, and GoogleNet) to process the data and forecast COPD- related results. Our results demonstrate how machine learning models may effectively identify subtle patterns and connections in intricate biological datasets. We found that there were significant differences between the three CNN architectures\' performances in terms of recall, accuracy, and F1-score after extensive testing and analysis. The intricate details of their architectural designs and optimisation techniques were reflected in the unique strengths and weaknesses that each model demonstrated. Bar graphs and comparison charts were employed to aid in the visual depiction and comprehension of model performance, hence furnishing stakeholders with significant discernments regarding the predictive capacities of each algorithm. These graphic aids were effective tools for disseminating study findings and promoting processes for making decisions based on data.In addition, the comparison study let us pinpoint possible areas for model optimisation and improvement, which set the stage for further studies aiming at improving prediction accuracy and extrapolation to unknown data. Our study adds to the expanding body of knowledge on pulmonary medicine by clarifying the subtleties of COPD development and physiological responses in smoke-inhaled lab rats. It also highlights the revolutionary potential of machine learning in biomedical research.Going ahead, sustained cooperation between scientists, medical professionals, and Data scientists will be crucial to expanding our knowledge of the pathophysiology of COPD and creating cutting-edge treatments for the treatment of the illness. We may work to enhance patient outcomes and provide a better future for those with COPD and other respiratory illnesses by utilising the power of interdisciplinary approaches and state-of-the-art technologies. Finally, our research provides important new information for the predictive modelling of the development of COPD and physiological responses, highlighting the enormous potential of machine learning in explaining intricate biological processes. As we continue to explore the boundaries of biomedical research, we are dedicated to using cutting-edge techniques and teamwork to solve the complex problems caused by respiratory illnesses and open the door to revolutionary breakthroughs in medical care.
References
[1] Li, Q.Y., Huang, S.G., Wan, H.Y., Wu, H.C., Tong, Z.H.O.U., Min, L.I. and Deng, W.W., 2007. Effect of smoking cessation on airway inflammation of rats with chronic bronchitis. Chinese medical journal, 120(17), pp.1511-1516. [2] Xie, Y., Garban, H., Ng, C., Rajfer, J. and Gonzalez-Cadavid, N.F., 1997. Effect of long-term passive smoking on erectile function and penile nitric oxide synthase in the rat. The Journal of urology, 157(3), pp.1121-1126.S. Zhang, C. Zhu, J. K. O. Sin, and P. K. T. Mok, “A novel ultrathin elevated channel low-temperature poly-Si TFT,” IEEE Electron Device Lett., vol. 20, pp. 569–571, Nov. 1999. [3] Oda, H., Matsuzaki, H., Tokuhashi, Y., Wakabayashi, K., Uematsu, Y. and Iwahashi, M., 2004. Degeneration of intervertebral discs due to smoking: experimental assessment in a rat-smoking model. Journal of Orthopaedic Science, 9(2), pp.135-141. [4] Park, M.G., Ko, K.W., Oh, M.M., Bae, J.H., Kim, J.J. and Moon, D.G., 2012. Effects of smoking on plasma testosterone level and erectile function in rats. The journal of sexual medicine, 9(2), pp.472-481. [5] Graziano, M.J. and Dorough, H.W., 1984. Effect of cigarette smoking on hepatic biotransformations in rats. Toxicology and applied pharmacology, 75(2), pp.229-239. [6] Park, E.M., Park, Y.M. and Gwak, Y.S., 1998. Oxidative damage in tissues of rats exposed to cigarette smoke. Free Radical Biology and Medicine, 25(1), pp.79-86. [7] Mayyas, F. and Alzoubi, K.H., 2018. Cardiac effects of cigarette tobacco smoking in rat model of diabetes. Life sciences, 211, pp.279- 285. [8] Lee, J.H., Lee, D.S., Kim, E.K., Choe, K.H., Oh, Y.M., Shim, T.S., Kim, S.E., Lee, Y.S. and Lee, S.D., 2005. Simvastatin inhibits cigarette smoking–induced emphysema and pulmonary hypertension in rat lungs. American journal of respiratory and critical care medicine, 172(8), pp.987-993 [9] Kapawa, A., Giannakis, D., Tsoukanelis, K., Kanakas, N., Baltogiannis, D., Agapitos, E., Loutradis, D., Miyagawa, I. and Sofikitis, N., 2004. Effects of paternal cigarette smoking on testicular function, sperm fertilizing capacity, embryonic development, and blastocyst capacity for implantation in rats. Andrologia, 36(2), pp.57- 68. [10] Luchese, C., Pinton, S. and Nogueira, C.W., 2009. Brain and lungs of rats are differently affected by cigarette smoke exposure: antioxidant effect of an organoselenium compound. Pharmacological Research, 59(3), pp.194-201. [11] Benatti, B.B., César?Neto, J.B., Gonçalves, P.F., Sallum, E.A. and Nociti Jr, F.H., 2005. Smoking affects the self?healing capacity of periodontal tissues. A histological study in the rat. European journal of oral sciences, 113(5), pp.400-403. [12] Dhabuwala, C.B., Dunbar, J.C., Li, H., Rajpurkar, A. and Jiang, Y., 2002. Cigarette smoking induces apoptosis in rat testis. Journal of Environmental Pathology, Toxicology and Oncology, 21(3). [13] Mayyas, F. and Alzoubi, K.H., 2019. Impact of cigarette smoking on kidney inflammation and fibrosis in diabetic rats. Inhalation toxicology, 31(2), pp.45-51. [14] Zhu, B.Q., Sun, Y.P., Sievers, R.E., Glantz, S., Parmley, W.W. and Wolfe, C.L., 1994. Exposure to environmental tobacco smoke increases myocardial infarct size in rats. Circulation, 89(3), pp.1282- 1290. [15] Hamosh, M., Shechter, Y. and Hamosh, P., 1979. Effect of tobacco smoke on the metabolism of rat lung. Archives of Environmental Health: An International Journal, 34(1), pp.17-23. [16] Small, E., Shah, H.P., Davenport, J.J., Geier, J.E., Yavarovich, K.R., Yamada, H., Sabarinath, S.N., Derendorf, H., Pauly, J.R., Gold, M.S. and Bruijnzeel, A.W., 2010. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology, 208, pp.143-158. [17] Anbarasi, K., Vani, G., Balakrishna, K. and Devi, C.S., 2006. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life sciences, 78(12), pp.1378-1384. [18] Dündar, M., Kocak, I. and Culhaci, N., 2004. Effects of long?term passive smoking on the diameter of glomeruli in rats: histopathological evaluation. Nephrology, 9(2), pp.53-57. [19] Miri-Moghaddam, E., Mirzaei, R., Arab, M.R. and Kaikha, S., 2014. The effects of water pipe smoking on hematological parameters in rats. International Journal of Hematology-Oncology and Stem Cell Research, 8(3), p.37. [20] Heidary, F., Ahmadi, R. and Lotfi, A., 2012. The effects of cigarette or hookah smoking on serum levels of LH, FSH or testosterone in male rats. In International Conference on Medical, Biological and Pharmaceutical Sciences (Vol. 12, pp. 103-105). [21] Yaffe, B., Cushin, B.J. and Strauch, B., 1984. Effect of cigarette smoking on experimental microvascular anastomoses. Microsurgery, 5(2), pp.70-72. [22] Khanna, A., Guo, M., Mehra, M. and Royal Iii, W., 2013. Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. Journal of neuroimmunology, 254(1-2), pp.69-75. [23] Ypsilantis, P., Politou, M., Anagnostopoulos, C., Kortsaris, A. and Simopoulos, C., 2012. A rat model of cigarette smoke abuse liability. Comparative medicine, 62(5), pp.395-399. [24] Costello, M.R., Reynaga, D.D., Mojica, C.Y., Zaveri, N.T., Belluzzi, J.D. and Leslie, F.M., 2014. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology, 39(8), pp.1843-1851. [25] Mohamed, M., Sulaiman, S.A., Jaafar, H. and Sirajudeen, K.N.S., 2011. Antioxidant protective effect of honey in cigarette smoke-induced testicular damage in rats. International Journal of Molecular Sciences, 12(9), pp.5508-5521. [26] Sorge, R.E. and Clarke, P.B., 2009. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. Journal of Pharmacology and Experimental Therapeutics, 330(2), pp.633-640. [27] Duarte, D.R., Oliveira, L.C., Minicucci, M.F., Azevedo, P.S., Matsubara, B.B., Matsubara, L.S., Campana, Á.O., Paiva, S.A. and Zornoff, L.A., 2009. Effects of the administration of beta-blockers on ventricular remodeling induced by cigarette smoking in rats. Arquivos brasileiros de cardiologia, 92, pp.479-483. [28] Carvalho, M.D., Benatti, B.B., César?Neto, J.B., Nociti Jr, F.H., da Rocha Nogueira Filho, G., Casati, M.Z. and Sallum, E.A., 2006. Effect of cigarette smoke inhalation and estrogen deficiency on bone healing around titanium implants: a histometric study in rats. Journal of periodontology, 77(4), pp.599-605. [29] Gomita, Y., Furuno, K., Eto, K., Okazaki, M., Suemaru, K. and Araki, Y., 1991. Effect of cigarette smoking on theophylline pharmacokinetics in rats. Journal of pharmacy and pharmacology, 43(9), pp.621-62 [30] Ma, L., Chow, J.Y.C. and Cho, C.H., 1999. Cigarette smoking delays ulcer healing: role of constitutive nitric oxide synthase in rat stomach. American Journal of Physiology-Gastrointestinal and Liver Physiology, 276(1), pp.G238-G248. [31] Cendon, S.P., Battlehner, C., Lorenzi-Filho, G., Dohlnikoff, M., Pereira, P.M., Conceição, G.M.S., Beppu, O.S. and Saldiva, P.H.N., 1997. Pulmonary emphysema induced by passive smoking: an experimental study in rats. Brazilian Journal of Medical and Biological Research, 30, pp.1241-1247. [32] netiga, Y., Matsuzaki, H., Tokuhasi, Y., Okawa, A., Uematu, Y., Nishimura, T. and Oda, H., 2006. Histological changes in intervertebral discs after smoking cessation: experimental study using a rat passive smoking model. Journal of Orthopaedic Science, 11(2), pp.191-197. [33] Harris, A.C., Mattson, C., LeSage, M.G., Keyler, D.E. and Pentel, P.R., 2010. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacology Biochemistry and Behavior, 96(2), pp.217-227. [34] Pittilo, R.M., Mackie, I.J., Rowles, P.M., Machin, S.J. and Woolf, N., 1982. Effects of cigarette smoking on the ultrastructure of rat thoracic aorta and its ability to produce prostacyclin. Thrombosis and Haemostasis, 48(05), pp.173-176. [35] César?Neto, J.B., Benatti, B.B., Sallum, E.A., Sallum, A.W. and Nociti Jr, F.H., 2005. Bone filling around titanium implants may benefit from smoking cessation: a histologic study in rats. Journal of periodontology, 76(9), pp.1476-1481. [36] Huh, J.W., Kim, S.Y., Lee, J.H., Lee, J.S., Van Ta, Q., Kim, M., Oh, Y.M., Lee, Y.S. and Lee, S.D., 2011. Bone marrow cells repair cigarette smoke-induced emphysema in rats. American Journal of Physiology-Lung Cellular and Molecular Physiology, 301(3), pp.L255- L266. [37] Kershbaum, A., Pappajohn, D.J., Bellet, S., Hirabayashi, M. and Shafiiha, H., 1968. Effect of smoking and nicotine on adrenocortical secretion. Jama, 203(4), pp.275-278. [38] Giorgetti, A.P.O., Neto, J.B.C., Ruiz, K.G.S., Casati, M.Z., Sallum, E.A. and Nociti Jr, F.H., 2010. Cigarette smoke inhalation modulates gene expression in sites of bone healing: a study in rats. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 110(4), pp.447-452. [39] Ortu?, C., 2003. Scanning electron microscopic findings in respiratory nasal mucosa following cigarette smoke exposure in rats. Annals of Anatomy-Anatomischer Anzeiger, 185(3), pp.207-210. [40] Lan, M.Y., Ho, C.Y., Lee, T.C. and Yang, A.H., 2007. Cigarette smoke extract induces cytotoxicity on human nasal epithelial cells. American journal of rhinology, 21(2), pp.218-223. [41] Khalmuratova, R., Kim, D.W. and Jeon, S.Y., 2011. Effect of dexamethasone on wound healing of the septal mucosa in the rat. American journal of rhinology & allergy, 25(3), pp.e112-e116. [42] Vidié, B., Rana, M.W. and Bhagat, B.D., 1974. Reversible damage of rat upper respiratory tract caused by cigarette smoke. Archives of Otolaryngology, 99(2), pp.110-113. [43] Mulligan, R.M., Atkinson, C., Vertegel, A.A., Reukov, V. and Schlosser, R.J., 2009. Cigarette smoke extract stimulates interleukin-8 production in human airway epithelium and is attenuated by superoxide dismutase in vitro. American journal of rhinology & allergy, 23(6), pp.e1-e4. [44] Joo, Y.H., Jeon, S.Y., An, H.J., Cho, H.J., Kim, J.H., Jung, M.H., Kim, R.B., Park, J.J. and Kim, S.W., 2019. Establishment and verification of a mouse model of nasal wound healing. The Laryngoscope, 129(8), pp.E266-E271. [45] Galeazzi, F., Blennerhassett, P.A., Qiu, B., O\'byrne, P.M. and Collins, S.M., 1999. Cigarette smoke aggravates experimental colitis in rats. Gastroenterology, 117(4), pp.877-883. [46] Drath, D.B., Harper, A., Gharibian, J., Karnovsky, M.L. and Huber, G.L., 1978. The effect of tobacco smoke on the metabolism and function of rat alveolar macrophages. Journal of Cellular Physiology, 95(1), pp.105-113.
Copyright
Copyright © 2024 Vinay Kumar Tripathi, Shubham Tripathi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63794
Publish Date : 2024-07-29
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

